Leading the Industry on Federal Traceability Issues
HDA and the healthcare distribution industry have led on federal traceability issues for nearly two decades. This work culminated in the passage of the Drug Supply Chain Security Act (DSCSA) in 2013. The DSCSA has fundamentally changed the way pharmaceutical products and their associated data move in tandem through the supply chain, while increasing safety and security for patients.
This groundbreaking law:
Replaced a 50-state patchwork of pedigree requirements with one federal solution. (A pedigree refers to the “product ownership” information associated with a drug as it travels through the supply chain.)
Clarified and consolidated supply chain regulations, increasing overall supply chain security — and ultimately patient safety.
Strengthened distributor licensure standards across the United States.
Established new processes for identifying suspect and illegitimate products in the supply chain.

HDA Guide for Submitting a DSCSA Waiver, Exception or Exemption Request
Companies seeking additional time to finalize DSCSA-compliant systems can file a Waiver, Exemption and Exception (WEE) request, which FDA will evaluate on an individual basis.
Download HDA’s newly published guide for submitting a WEE request here.

HDA VRS Provider Network
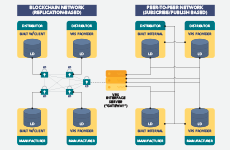
The Verification Router Service Provider Network (VRS PN) is an HDA service offering that provides a forum for pharmaceutical supply chain thought leaders on technology-based solutions and innovation to network with peers and regulatory officials, participate in meetings and exchange information that advances ongoing interoperability for DSCSA compliance.
The VRS is an industry-developed solution that allows companies to verify products in compliance with the 2019 DSCSA requirement for serialized saleable returns. It enables verification of product identifiers for suspect or illegitimate product investigations, exception processing, status checks and saleable returns.

Prepare for DSCSA Exceptions and Data Errors with HDA Guides
HDA’s Exceptions Handling Guidelines for the DSCSA address the exceptions that may arise when passing or failing to pass DSCSA-required information to authorized trading partners. Published addendums to the guidelines share best practices for distributors and manufacturers to manage communications and correct data errors.
FDA's Phased Implementation and Exemptions Policy
 | HDA supported the FDA’s decision to grant a phased implementation approach for compliance and their actions to permit specific “Waivers, Exceptions and Exemptions” requests for supply chain entities still building out systems for the enhanced drug distribution security requirements in the DSCSA. On August 27, 2025, the distribution industry reached their implementation deadline with minimal disruption to the supply chain. Read HDA’s full statement on this milestone here. |
At the Nexus of DSCSA Implementation
DSCSA implementation is a perfect example of how the distribution industry’s collaborative spirit and logistics expertise benefit the entire supply chain. By extension, through every milestone of DSCSA implementation, HDA has served as a convener and intermediary between regulatory and industry stakeholders; voiced the interests of its members that are leading implementation efforts for the industry; assembled supply chain stakeholders to develop collaborative, industry-driven solutions to complex challenges; and spearheaded educational offerings, including an annual Traceability Seminar and a cross-sector dispenser working group, to engage trading partners by providing compliance information and support.
Highlights of HDA Activities to Support Implementation
Resources
The resources below address the industry’s approach on federal traceability issues and DSCSA implementation.
- Submitting a Waiver, Exception or Exemption to FDA
- Fact Sheet: The Drug Supply Chain Security Act
- 2022 Serialization Readiness Survey
- Exceptions Handling Guidelines for DSCSA
- HDA Guidelines for Bar Coding in the Pharmaceutical Supply Chain
- HDA Saleable Returns Pilot Study: 2019 Compliance Scenarios
- HDA Standard Pharmaceutical Product and Medical Device Information (New Product Form)
- Imported Medicines, at What Cost? (Infographic)
- Manufacturer Data Quality: Best Practice Considerations for DSCSA
- The Risks and Realities of Commercial Drug Importation
- HDA Letter to Commissioner Makary
- HDA Letter to HHS Secretary Kennedy
- Congressional Letter to FDA on DSCSA to Avoid Drug Supply Chain Disruptions
- HDA Comments to FDA on Industry Concerns Ahead of DSCSA Deadline
- HDA Comments to FDA on WEE Clarifications
- HDA Comments to FDA on EDDS Final Guidance
- HDA Comments to FDA on State of Industry’s Readiness for DSCSA Deadline
- Congressional Letter to FDA on DSCSA
- HDA Comments to FDA on the State of DSCSA Readiness
- HDA Statement for the FDA DSCSA Public Meeting Implementation and Readiness Efforts for 2023
- HDA Comments on FDA's Proposed National Standards for Licensure of Distributors and 3PLs
- HDA Comments on the FDA Revised Guidance: DSCSA Standards for the Interoperable Exchange of Information for Tracing of Certain Human, Finished, Prescription Drugs Guidance for Industry
- HDA Comments on the FDA Revised Guidance: Identifying Trading Partners Under the DSCSA
- HDA Comments on FDA’s Verification Draft Guidance
- HDA Comments to Follow-up on FDA’s November 16, 2021, DSCSA Public Meeting
- HDA Statement to FDA on November 16, 2021, DSCSA Public Meeting
Our Advocacy Work on Behalf of Members
HDA advocates for pharmaceutical distributors by working with policymakers and industry stakeholders to advance policies, standards and practices that strengthen the healthcare supply chain. HDA members have a powerful, unique voice that helps to shape public policy, educate lawmakers and provide critical insights.
| Not a member? Join today. |